TherapySelector app for iPhone and iPad
The HCV Therapy Selector is uniquely handy and complete source of information intended for the physician and his patient with hepatitis C to select the best fitting treatment.
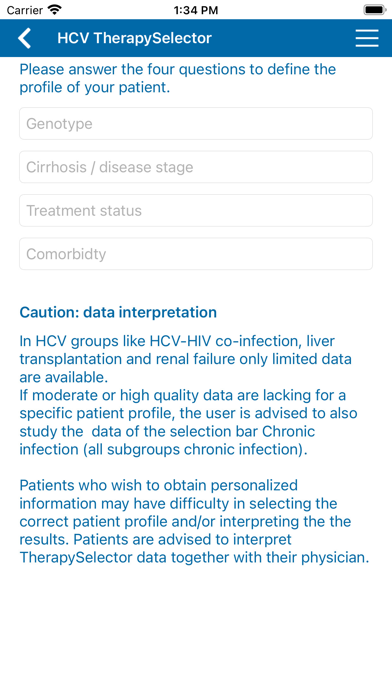
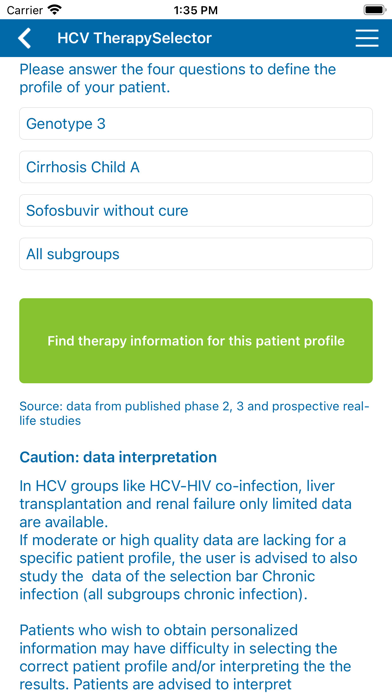
Recommendations for specific patient profiles based on genotype, previous treatment history and cirrhosis stage are given.
In addition the HCV Therapy Selector provides evidence-basedí data for more than 60 patient profiles about the chance of cure of hepatitis C infection and treatment-specific information about side effects, drug interactions, costs and reimbursement.
The HCV Therapy Selector is developed through collaboration between LiverDoc, Erasmus Medical Center Rotterdam, Netherlands Association for the Study of the Liver (NASL), and ExpertDoc. LiverDoc and ExpertDoc make clinical decision support products to bring academic knowledge to the busy physician and informed choice to the patient. Funding of the HCV Therapy Selector has come from LiverDocís own resources without contributions of the pharmaceutical industry.
Patient-profile specific knowledge from 3 (inter)national guidelines comprising 302 pages and more than 150 publications of antiviral therapy hepatitis C can be assessed within 1 minute; all information is updated twice a year.
Patient characteristics crucial for treatment decision making are HCV genotype (1-6), stage of cirrhosis (no/Child A/Child B-C), previous treatment exposure (no/(peg)interferon/sofosbuvir), thereby creating 54 patient profiles in the chronic mono- & co-infection group.
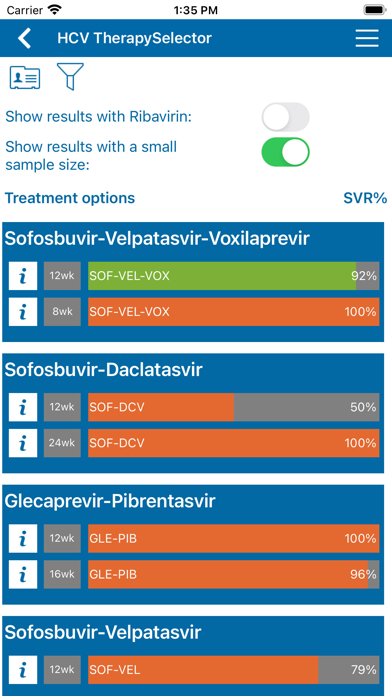
All 13 treatment regimens approved by the FDA and European Medicines Agency by September 2016 were included.
The published preregistration clinical trials as well as real-life postregistration studies available per September 2016 for these regimens were assessed for data on SVR rates (HCV RNA undetectable 12-24 weeks after stopping antiviral therapy).
For each of the 10 treatment schedules, data (total number of patients treated and evaluable for SVR 12 or SVR 24, and total number of patients with SVR per patient profile) were extracted from the publications. Often supplementary data were obtained . After an internal and external check the data were entered into a database. A program pooled the patient profile specific data derived from various studies, calculated the SVR percentages and assigned a quality label to the result.
Quality of evidence was graded according to a simple system: ëhighí (at least 80 patients, data from two studies), ëmoderateí (at least 40 patients or 80 patient data from one study) and ëlowí (less than 40 patients).
Adverse effects are shown if such effects are clinically relevant by its severity or by a higher frequency than in placebo-controlled trials. This restrictive way of information intends to increase its clarity and may reduce the nocebo effect of comprehensive information.
Costs and reimbursement information are derived from robust sources like www.hepatitisc.uw.edu (USA), Journal Officiel de la RÈpublique FranÁaise (FR) and www.medicijnkosten.nl (NL).
The data were collected by research fellows at the Erasmus Medical Center in Rotterdam (R. Maan, M. van Tilborg) and a senior specialist (Dr. S.W. Schalm, em Professor of Clinical Hepatology and director of LiverDoc), and authorized by an hepatitis expert (Dr. R.J. de Knegt) on behalf of the Netherlands Association for the Study of the Liver (NASL).